Which of the Following Represents a Hydrogen Displacement Reaction
Considering the options given in the question above only option A fits into the discription of an acid base reaction as the product contains salt NaBr and water H₂O only. Oxygen displaces hydrogen in the ammonia making it a displacement reaction.
Chemical Reactions Chemical Reactions Are Described By Chemical
2 Als 3H2SO4aq Al2SO43aq 3 H2g.
. 2 H2g O2g 2 H2Ol B. It dictates that the number of atoms of each element must be the same on both sides of a chemical equation. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding.
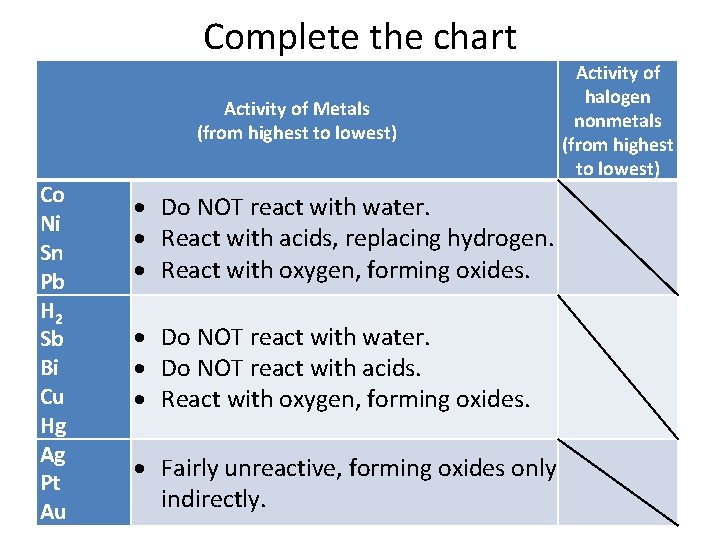
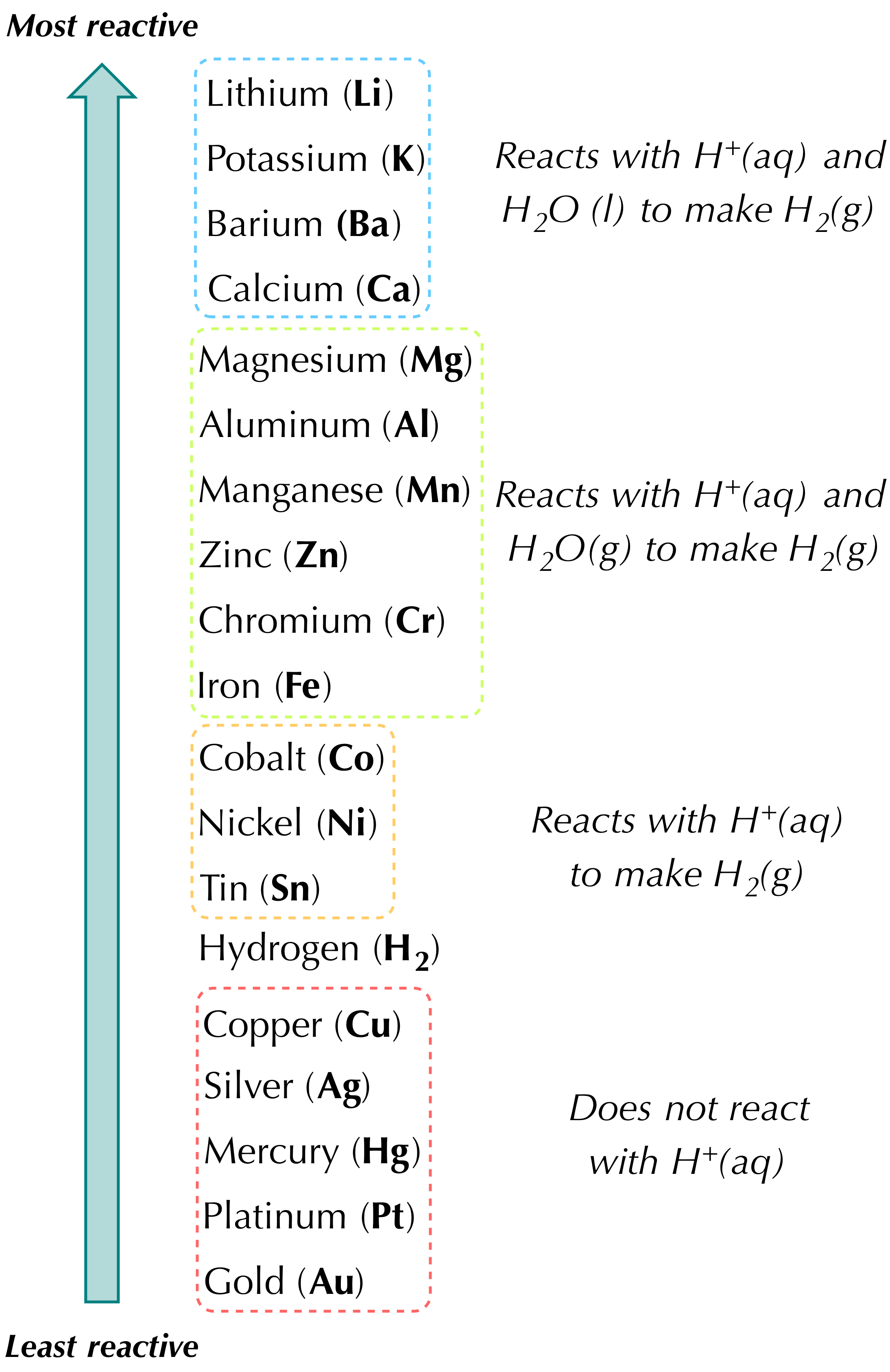
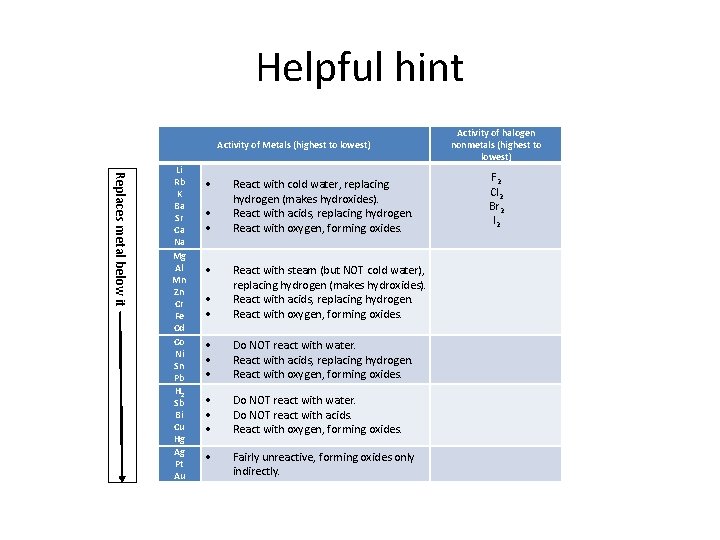
CK-12 Some metals are so reactive that they are capable of replacing the hydrogen in water. Displacement reactions will only occur in metals above iron in the reactivity series. A 2C2H6g 7O2g 4CO2g 6H2Ol B 2KBraq Cl2g 2KClaq Br2l C N2g 3H2g 2NH3g D CaBr2aq H2SO4aq CaSO4s 2HBrg E 2Als 3H2SO4aq Al2SO43aq 3H2g 27.
The reaction of formation of hydrogen chloride from hydrogen and chloride represents the following type of reaction. Fe2O3s 2Als Al2O3s 2Fes is an example of a A. 2KBraq Cl2g.
Click in the answer box to open the symbol palette. 4CO2g 6H2Ol B. 2NaOHaq H2g E.
CaBr2 aq H2SO4 aq CaSO4 s 2HBr g 3. Decomposition reaction as well as displacement reaction. Redox reaction as well as displacement reaction c.
2K Br aq Cl2 g 2KCl aq Brz 1 C. The reaction of formation of hydrogen chloride from hydrogen and chloride represents following type of chemical reaction. A less reactive metal displaces a more reactive metal from its compound.
Which of the following represents a hydrogen displacement reaction. Double displacement reaction C. What type of reaction is the following.
The reaction provided is a mixture of displacement and redox reaction. Which of the following represents a hydrogen displacement reaction. A displaystyle ce A and.
Which of the following represents a hydrogen displacement reaction. Silver Nitrate Sodium Chloride. 2KBr aqCl2 g 2KCI aq Br20l C.
Calcium zinc nitrate goes to. CaBr2aq H2SO4aq CaSO4s 2 HBrg C. Displacement only occurs when two of the same metals are reacted.
N2g 3H2g. N2 g 3H2 g 2NH3 g D. Which among the following reactions represents the displacement of less reactive metal from its salt solution by a more reactive metal.
Magnesium metal reacts with hydrochloric acid to form magnesium chloride and hydrogen gas. CaBr2aq H2SO4aq. Short Answer---6 POINTS EACH -60 POINTS MAXIMUM.
2C2Hs g702 g - 4C02 g 6H20 1 B. Combination reaction as well as double displacement reaction b. 2C2H6g 7O2g.
What happens when dilute hydrochloric acid is added to iron fillings. N2g 3H2g 2NH3g D. CaBr2 aqH2S04 aqCaS04 s 2HBr g D.
Include states of matter in your answer. Which one of the following metals replaces hydrogen from acid. 2Al s3H2SO4 aq - Al2 S04s aq3H2 B 6.
2 KBraq Cl2g 2 KClaq Br2l E. 2 KNO3s 2 KNO2s O2g D. CaBr2aq H2SO4aq CaSO4s 2HBrg E.
Nitrogen gets oxidized and oxygen is reduced resulting in a redox reaction. In a hydrogen replacement reaction the hydrogen in the acid is replaced by an active metal. 4pt Which of the following will occur when a solution of Pb NOa aq is mixed with a solution of.
3pt Which of the following represents a hydrogen displacement reaction. C displaystyle ce C is an anion. 2C2H6g 7O2g 4CO2g 6H2Ol B.
Magnesium metal displaces Hydrogen from HCl. You are given the following chemical reaction. A Als Cl2g AlCl3s b PbNO32aq K2CrO4aq PbCrO4s KNO3aq c Lis H2Ol LiOHaq H2g.
B displaystyle ce B are different metals or any element that forms cation like hydrogen and. 3Fes 4H 2 Og Fe 3 O 4 s 4H 2 g. Chemistry QA Library Which of the following represents a double-displacement reaction.
Zinc metal reacts with hydrochloric acid to give off hydrogen gas in a single-displacement reaction. This is a reaction in which an acid reacts with a base to produce salt and water only. 2KBraq Cl2g 2KClaq Br2l C.
2KClaq Br2l C. 2Als 3H2SO4aq Al2SO43aq 3H2g Answer. A NaClaqAgNO_3aqlongrightarrow NaNO_3aqAgCl darr b 2MgsO_2glongrightarrow2MgOs c CaCO_3s-Delta_ CaO sCO_2g d ZnsH_2SO_4aqlongrightarrow ZnSO_4aqH_2g.
Chemistry questions and answers. CaOH 2 s 2 HNO 3 aq CaNO 3 2 aq 2 H 2 Ol A Combination reaction D Disproportionation reaction B Acid-base neutralization reaction E Combustion reaction C Hydrogen displacement reaction Ans. C Hydrogen displacement reaction Ans.
Balance each of the following skeletal equations. Write a balanced equation to represent this reaction. Which of the following statements about the given reaction are correct.
This reaction represents a displacement reaction. Acidbase reaction is other wise known as neutralization reaction. Which of the following represents a hydrogen displacement reaction.
D Double displacement reaction. 2Als 3H2SO4aq Al2SO43aq 3H2g Which of the following represents a combustion reaction. It dictates that the number of molecules on each side of chemical equation must be the dame.
It can be represented generically as. A BC AC B displaystyle ce A BC - AC B where either. 2 points Which of the following represents a hydrogen displacement reaction.
Which of the following equation represents single displacement reaction. G 702 g 4CO2 g 6H2O 1 B. It states that the mass of the reactants must remain constant in order for a chemical reaction to proceed.
2Nas 2H2Ol. Double displacement reaction as well as redox reaction d.

Write The Balanced Chemical Equation For The Following And Identify The Type Of Reaction In Each Case A Potassium Bromide Aq Barium Iodide Aq Potassium Iodide Aq Barium Bromide S B Zinc Carbonate S Zinc
Chemical Reactions Chemical Reactions Are Described By Chemical

Solved Which Of The Following Represents A Hydrogen Chegg Com

Chemistry Hydrogen Reaction And Chemical Equations 9 Of 38 Types Of Reactions Iii Youtube
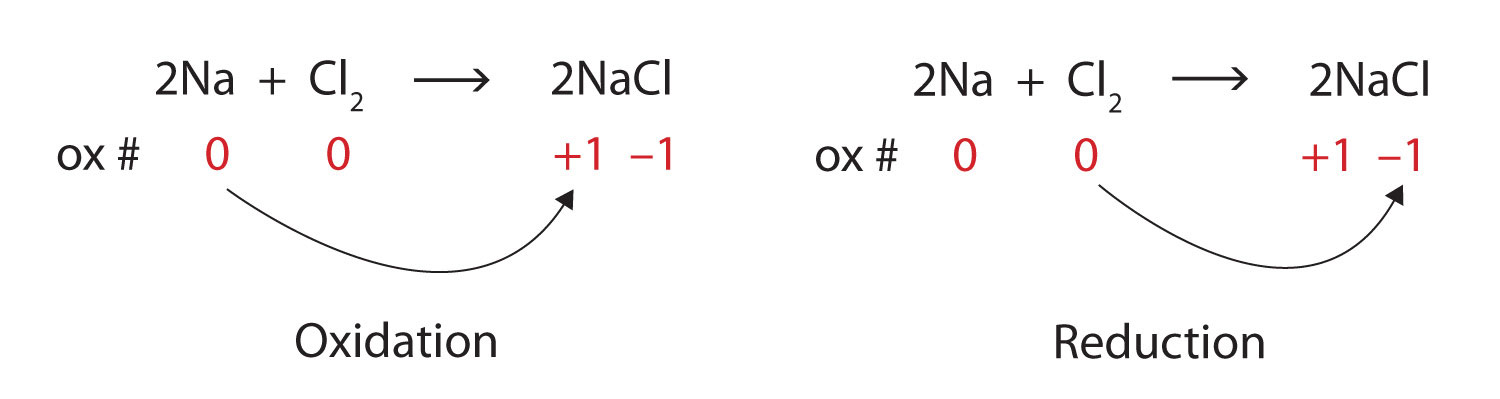
Chemical Reactions And Equations

Chemistry Of Matches P4s3 Kclo3 P2o5 Kcl So2 D Tetraphosphorus Ppt Download

Bell Ringer Complete And Balance Each Of The Following Synthesis Reactions By Writing Chemical Equations A Na O2 B Mg F2 A 4na Ppt Download

Chemistry Hydrogen Reaction And Chemical Equations 9 Of 38 Types Of Reactions Iii Youtube
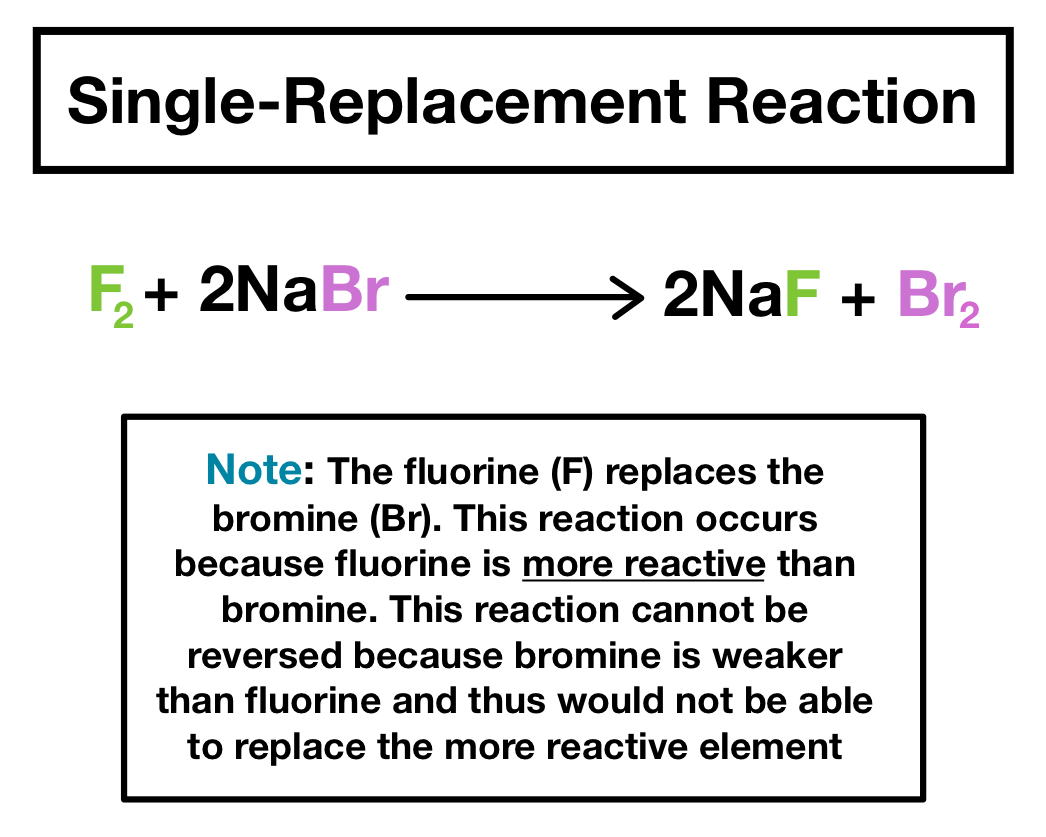
Single Replacement Reactions Definition Examples Expii
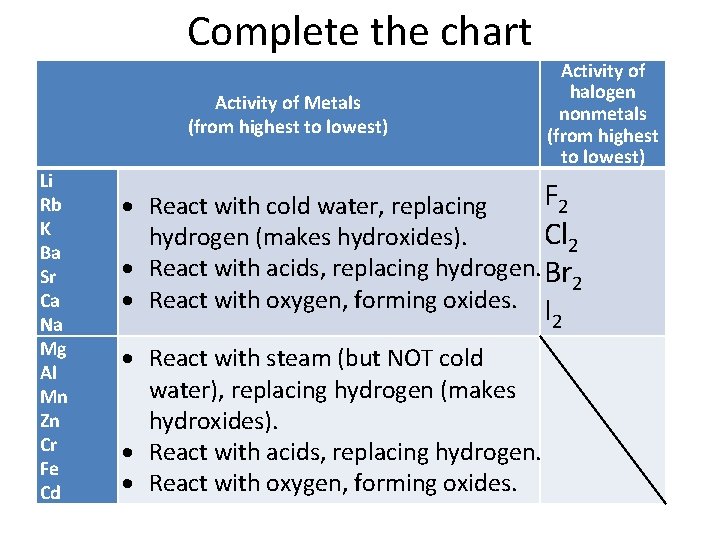
Chemical Reactions Chemical Reactions Are Described By Chemical

A Chemical Reaction Is The Process By Which One Or More Substances Are Changed Into One Or More Different Substances In Any Chemical Reaction The Original Ppt Download

Chemical Reactions Ck 12 Foundation
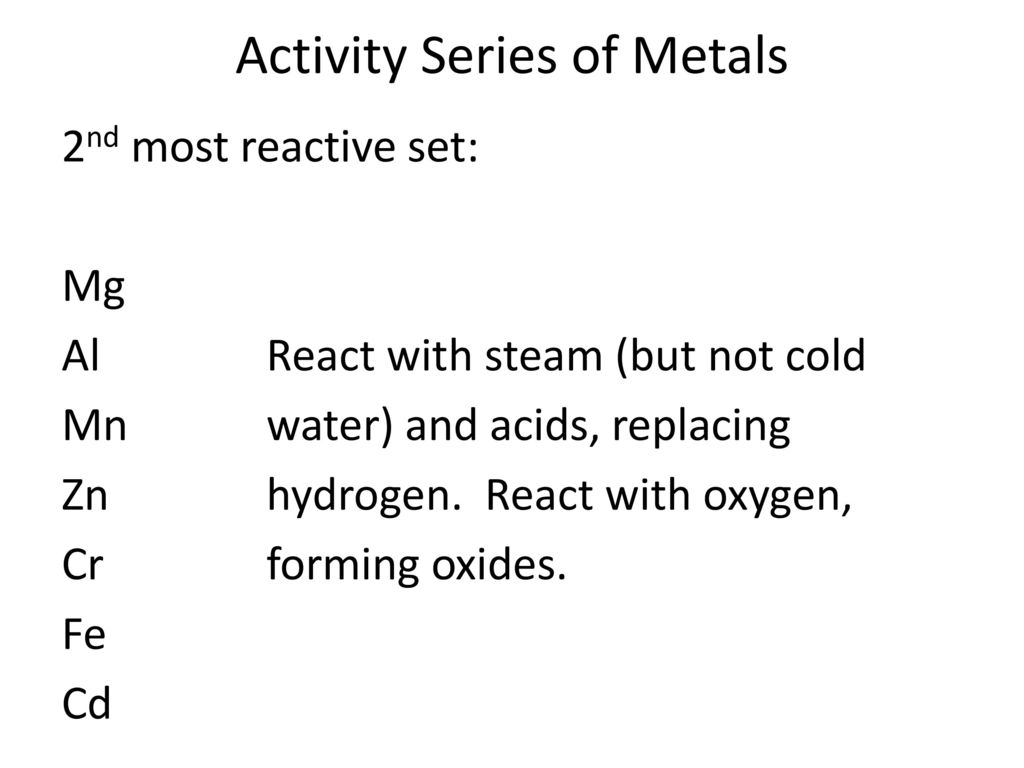
Bell Ringer Complete And Balance Each Of The Following Synthesis Reactions By Writing Chemical Equations A Na O2 B Mg F2 A 4na Ppt Download

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations Free Pdf

Type Of Reaction For H2so4 Zn Znso4 H2 Youtube

Chemical Equations Reactions Ppt Video Online Download

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations Free Pdf
Comments
Post a Comment